November 24, 2010
By Toby Bilanow
For centuries, scientists had few clues about the inner workings of the brain. The three pounds of nerve tissue that give us our thoughts and memories remained stubbornly out of reach. But all that is changing rapidly. Advances in imaging are unlocking the mysteries of the hundred billion interconnected nerve cells that shape our personalities and consciousness. They are also providing vital insights into just what happens when things go terribly wrong—as in Alzheimer’s disease—to rob us of our memories and selves.
Most of the time, in about 9 cases out of 10, doctors use their clinical judgment, along with medical histories, memory exams, and brain scans, to correctly identify Alzheimer’s disease. But diagnosis often comes years after the disease starts, when Alzheimer’s has already done irreparable damage to the brain. And a definitive diagnosis still requires an autopsy, where pathologists can examine first-hand the telltale plaques and tangles that mar the brains of those with the disease.
An imaging device or other test to diagnose Alzheimer’s at an earlier stage, before grave damage has been done, could be key to effectively treating, or even curing, the disease when effective therapeutic measures become available. Such a test could identify people at risk even before memory loss and other symptoms arise, when drugs and other therapies may be most effective. It might also allow doctors to monitor people with the disease to determine whether new, experimental treatments are working.
“Early detection may be critical for slowing, or even halting, the relentless downward progression of Alzheimer’s,” says Nobel laureate Dr. Paul Greengard, director of The Fisher Center for Alzheimer’s Research at The Rockefeller University in New York. “As we continue to unlock the basic underlying causes of the disease, it is vital that we come to understand the course of Alzheimer’s at its earliest stages.”
Mapping the Living Brain
We’ve come a long way since German physician Alois Alzheimer first peered into a light microscope a century ago to examine the shrunken brain of Auguste D., the middle-aged woman who died of the memory-robbing ailment that would later bear his name. There, magnified several hundred times, were the clumps of “senile plaque” that scientists would later identify as beta-amyloid, the toxic protein that builds up in the brains of Alzheimer’s patients. Speckled throughout were also the string-like tangles composed of the substance we now call tau.
Today, scientists around the world are using advanced, computerized imaging techniques and complex molecular markers to delve into the secrets of the living brain. They can view the brain at a molecular level inconceivable in Dr. Alzheimer’s day, and assess what goes wrong in an illness like Alzheimer’s.
Scanning methods like CT (computed tomography) and MRI (magnetic resonance imaging) can be used to identify minute structural changes in the brain. Researchers at Dartmouth Medical School recently used MRI, for example, to show that forgetful seniors have less gray matter, the outer brain layer crucial for thinking and memory, than their mentally alert peers.
A particularly promising form of MRI, called functional MRI, looks not just at brain structure but also at brain function. The technique can, for example, determine which parts of the brain are at work when we try to recall the name of our first-grade teacher or solve a long division problem. Studies are currently under way to determine if functional MRI can be combined with other imaging and genetic tests to identify patients at risk for future Alzheimer’s.
“As new therapies for Alzheimer’s disease enter the pipeline over the next five years, early diagnosis will become critical for patient selection,” says Dr. Jeffrey Petrella of Duke University. He recently completed a functional MRI study that found that a part of the brain that recalls personal memories may actually become uninhibited in people with incipient Alzheimer’s, impairing their ability to complete more focused everyday tasks.
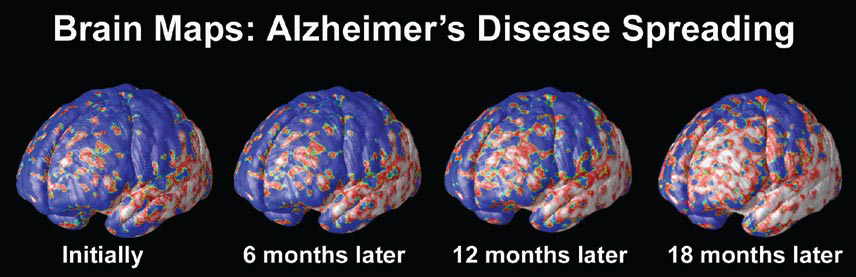
A Window Into the Brain
Other high-tech imaging techniques, like PET (positron emission tomography) and SPECT (single photon emission computed tomography), provide unique views into the living brain at work. Areas on the computer screen that light up red, for instance, may show parts of the brain that are actively absorbing a radioactive tracer, while blue areas may indicate brain areas that are dulled. Low activity in memory centers of the brain, for example, may detect Alzheimer’s at its earliest stages, years before symptoms such as forgetfulness appear.
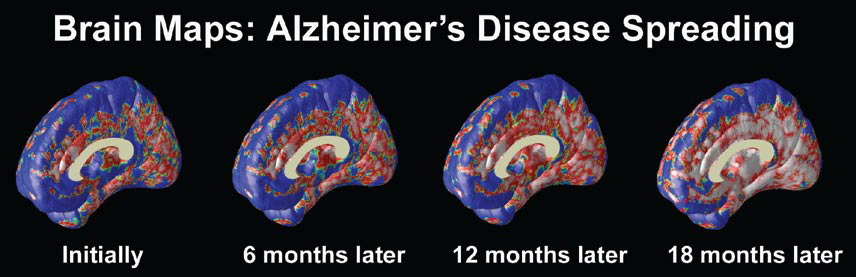
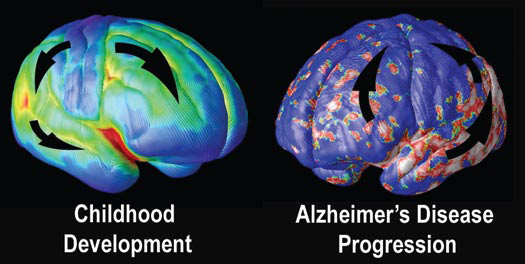
At the University of California at Los Angeles (UCLA), brain researcher Dr. Paul Thompson has created remarkable 3-D time-lapse images of brains affected by Alzheimer’s by overlaying images collected from various scanning techniques. Among the areas where cell death may first occur are in the hippocampus, the seahorse-shaped brain structures behind the ears that control memory processing. These are followed by other structures in the limbic system, affecting emotions, and by the frontal lobe areas that affect decision making and self-control.
“One of the diseases that really has a chance to be cured is Alzheimer’s,” says Dr. Thompson. “Imaging offers the opportunity to capture a detailed picture of what is going on in the brain of someone with Alzheimer’s over time. It can also tell you if the disease is being slowed down by a particular drug, or even by lifestyle factors like diet or exercise.”
A new imaging agent called Pittsburgh Compound B, developed at the University of Pittsburgh, is allowing doctors for the first time to visualize the buildup—or dissipation— of beta-amyloid in the living brain. The compound slips past the blood-brain barrier and attaches to plaque, where it appears as a brightly lit image on PET scans.
“For the first time, doctors may be able to tell definitively if treatment is working,” says Dr. Steven DeKosky, director of Pittsburgh’s Alzheimer’s Research Center. “If we can make a specific early diagnosis through imaging, then we can track the effectiveness of new drugs and other treatments as well.”
Another substance called FDDNP, developed at UCLA, attaches to plaques and tangles in the brain and is similarly visualized using PET scans. In a recent study, it proved very effective in distinguishing patients with mild cognitive impairment, a form of memory loss that often precedes Alzheimer’s.
Additional strategies are also under development. At the Institute for Neurodegenerative Disorders in New Haven, Conn., scientists are testing radioactive tracers in combination with SPECT to visualize the buildup of beta-amyloid plaque over time in people with early Alzheimer’s. Investigators also hope to test the response to treatment with vaccines and other treatments that may rid the brain of the toxic protein. Several other tracers targeting other brain proteins are in development with the goal of providing a more complete picture of brain changes that occur in AD.
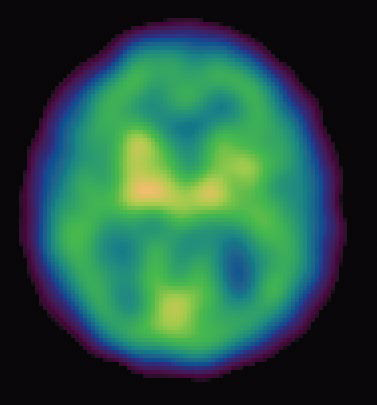
“Neuroimaging is a tool with extraordinary potential to gain a window into the brain chemistry in Alzheimer’s disease and to help to develop and monitor new therapies… enabling us to conduct the research important to both Parkinson’s and Alzheimer’s disease,” says Dr. Kenneth Marek, president and senior scientist, Institute for Neurodegenerative Disorder.
And at the University of Minnesota, doctors are studying a simple 60-second test called MEG (magnetoencephalography) that, like an EKG for the heart, measures magnetic fields emitted by the brain and which, in initial tests, detected early Alzheimer’s.
Although research on these and other techniques is just beginning, it may allow scientists to detect Alzheimer’s at its earliest stages, up to a decade or more before patients experience symptoms like memory loss.
Alzheimer’s “Fingerprints”
In addition to brain imaging, researchers have long sought to identify natural substances in the blood or body that distinguish Alzheimer’s from other forms of dementia. Scientists in New York are using cutting edge “proteomics” technology, which analyzes proteins in the cerebrospinal fluid that bathes the brain and spinal cord.
“Just as the human genome reflects the array of genes a person possesses, the ‘proteome’ is the vast collection of proteins expressed by those genes,” explains Cornell University professor Dr. Kelvin Lee. Using detailed image analysis and complex computer and statistical analyses, he and colleagues have identified a group of 23 proteins in the spinal fluid that may be unique to Alzheimer’s disease and serve as a unique “fingerprint” that could one day lead to a test to diagnose Alzheimer’s at its earliest stages.
“You might have a promising treatment for the disease, but how can you know for sure that it’s impacting on the underlying pathology, rather than just easing outward symptoms, as most of the drugs that we have now do?” says Dr. Norman Relkin of Weill Cornell Medical College, who is involved in the current research. “We are hopeful that by monitoring changes in these cerebrospinal biomarkers, we can actually track the effectiveness—or lack thereof—of experimental drugs.”
At Stanford University, scientists recently identified a set of 18 proteins in the blood that may signal early Alzheimer’s and predict which patients with mild cognitive impairment go on to develop full-blown Alzheimer’s. Still, it will likely be several years before results are confirmed.
Other early screening tests, some surprisingly low-tech, are also being tested. In one recent report, people with poor memory who misidentified more than 2 of 10 common scents—strawberry, pineapple, lilac, clove, menthol, lemon, smoke, natural gas, soap, and leather— were five times more likely to progress to Alzheimer’s years later than those who retained their olfactory sense. This “sniff test” will require further confirmation, but in initial tests, it performed as well as memory exams and MRI scans in determining which people would go on to develop Alzheimer’s. Researchers speculate that the same processes that destroy brain cells vital for memory may also affect those crucial for scent. Other scientists are looking into a “skin test” that measures chemical changes in the skin that may signal early Alzheimer’s.
An ongoing public-private research collaboration called the Alzheimer’s Disease Neuroimaging Initiative, funded in part by the National Institutes of Health, is working with medical centers across north America to identify additional biomarkers for Alzheimer’s disease. Researchers will examine more than 800 men and women, ranging in age from 55 to 90, using PET scans, MRI, and blood and spinal fluid tests in an attempt to identify biomarkers that may be an early warning sign of Alzheimer’s.
“Earlier diagnosis means earlier treatment,” says the Fisher Center’s Dr. Greengard. “And the more we understand about the basic causes of Alzheimer’s, the closer we move to a cure.”
Figures 1, 2, 3 Courtesy of Dr. Paul Thompson, Professor of Neurology, UCLA School of Medicine
Source: www.ALZinfo.org. Author: Toby Bilanow, Preserving Your Memory: The Magazine of Health and Hope; Winter 2007.











